I'd like to add some of my thoughts about the article and some of the comments above. There are many good points brought up in all of this discussion, and I do agree that steam is the most cost effective and simple sterilization methods available. However, I'd like to list some points below about EO sterilization that I believe could use some clarification. These points are listed in the order of how they appear in the article and then in the comments.
* EO is the biggest method of industrial sterilization in the U.S. and it is generally considered to be approximately 52% of the market. (Gamma ~37%, Ebeam ~8%, and the other sterilization methods represent the remaining ~3%.) In addition, the percentage of products processed in industry by EO have grown in the last ten or so years by ~2-3%. (Please remember that these numbers are specific to the U.S., and to industrial sterilization market. I do not know what these figures are outside the U.S. for industrial sterilization, or what they are in healthcare facilities. If someone else knows this information, it would be great if you would please share it.)
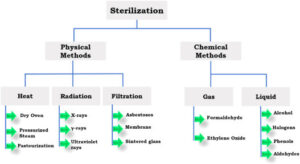
* The conventional EO sterilization process includes three steps - Preconditioning, Sterilization, & Aeration. In the All-In-One (AIO) process this is accomplished on one step.
* With the conventional 3 phase EO sterilization process, the typical Sterilization cycle time is between 8-12 hours. In addition to that, the typical preconditioning time is 8-12 hours, and the aeration time can be as short as 8-12 hours. In the AIO process, the entire processing time is often in the 18-22 hour range.
* To repeat something stated above for emphasis, the process of heating and humidifying the load is called preconditioning when it's performed outside of the sterilizer chamber, and when it's performed inside the sterilizer chamber its call conditioning. The typical conventional 3-phase sterilization process uses both preconditioning and conditioning. The AIO process uses only conditioning.
* Liquid EO is changed to gaseous EO by a volatilizer before it enters the chamber.
* Again to repeat a point made above, but with a slightly different slant, the preconditioning, sterilization, and aeration are all most important parts of the process.
* To reiterate a prior statement, aeration can be as short 8-12 hours, and in some cases it could last for extended periods.
* EO sterilizers can be 100 cubic meters in size or larger.
* The residues that are absorbed and adsorbed onto the devices processed with EO are required to be aerated to below government specified limits before they can be used on patients.
* Flexible scopes can be, and often are, processed with EO.
* The costs for maintenance, servicing, and consumables with EO are more than steam, but are often less than for other sterilization methods.
* EO is the most material friendly sterilant in existence today. In an oversimplified way, the only products that typically cannot be processed with EO are 1) liquids, and 2) ones in non-breathable packaging.
* Since EO is an organic substance it is absorbed (and adsorbed) by plastics and some other materials.
* It does state in the ISO documents that other methods of sterilization should be used before choosing EO. However, the reality of the situation is that EO may often be the only method of sterilization and/or the preferred method of sterilization for many products. That is why it is the biggest method of industrial sterilization in use today.
* EO sterilization, if properly performed, is an environmentally friendly sterilization method since the regulations require that, if sufficient volumes of EO are used, at least 99% of it must be removed before it can be exhausted to the atmosphere.