On November 7 PMP News published the article, "How to Make the Choice between Ethylene Oxide or E-Beam Sterilization," by Freelance Journalist Camilla Andersson. It was also included in the Medical Packaging Community.
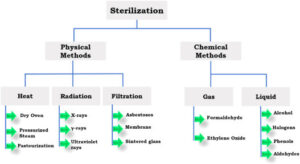
The article discussed the choice between Electron beam (E-beam) sterilization and ethylene oxide (EO).
PMP News received the following letter in response to that article:
Thanks for the good and interesting article. I especially appreciated the input by Bill Young. I’d like to take this opportunity to expand on some of the presented thoughts.
The article may have been a bit more interesting and appropriate if it was entitled “How to Make the Choice between Ethylene Oxide (EO) or Radiation Sterilization (gamma, E-beam, and X-ray).” I say that as it is perhaps the more appropriate and one of the most often asked questions of this nature. If that was the subject of the article, I may have had some additional input to what I’m going to state below, but since it is about the choice between EO and E-beam, I’ll confine most of my comments to the subject in this article.
First let me start by saying that I am neutral when it comes to choosing a sterilization method. All of the aforementioned sterilization methods, as well as some others, make excellent sterilants when used properly. It is generally accepted that the split between industrial sterilization methods is approximately 52% EO, 37% gamma, 8% E-beam, and the remainder are various other methods. With this in mind, and while there are some products that can be sterilized with either EO or E-beam, I would suggest to you that the percentage of those is somewhat low. In addition, if one were doing both EO and E-beam sterilization and wanted to combine them into one method for any one of various reasons, it would be more likely that all of the products could be processed with EO than all of the products could be processed by E-beam.
There was mention of the fact that E-beam is “suitable for a variety of materials.” There is a relatively large variety of materials that can be processed with E-beam, but there are also some which are not very compatible with that methodology. The corollary to this for EO is that it is the most material friendly method of sterilization, which is the primary reason for its large scale use. From a generic standpoint, the only two categories of products that cannot be processed with EO are liquids and devices in gas impermeable packages.
It is true that, of the major sterilization methods mentioned above, E-beam is the most rapid. From a practical standpoint, it is relatively common for contract E-beam sterilizers, for example, to turn loads around within a three day timeframe, although many also offer same day service, and next day service, as additional cost options. Whereas it is true that classical EO sterilization typically “takes days,” more modern methods allow for many products to be EO sterilized and released to market within approximately 18-24 hours. This is typically accomplished by doing all-in-one processing (preconditioning, sterilization, and aeration and performed in the sterilizer chamber) combined with parametric release. When this can be employed, it allows for products to be sterilized, certified sterile, and meet governmental limits for EO residues within one day.
Packaging for EO does require breathable packaging to allow the EO gas and relative humidity to get into and out of the package. While it is correct to state that “the product or package needs to tolerate vacuum and pressure changes,” the level of the vacuum and pressure changes, as well as the rate to achieve these vacuum and pressure changes, can be adjusted to allow for most of these issues to be overcome. In addition, temperatures for EO sterilization are generally considered to be relatively low, often less than 135 degrees F, which for the vast majority of products is not an issue. (Please keep in mind that studies done in tractor trailers in the warmer climates of the U.S. show that temperatures can often get to 140 degrees F.) Since breathable packaging is not required for products processed with E-beam, packaging can sometimes be less expensive (e.g., polyethylene bag.)
A good point in the article states that products that contain drugs are often not processed with e-beam. The primary reason for this is that they would have to be tested extensively to show that the post sterilized drug is still safe and efficacious. Products containing drugs often have the drugs purchased pre-sterilized, placed in gas impermeable packaging (e.g., hermetically sealed glass vials), and then placed with products that are EO processed. In this case the EO sterilizes the outside of the (gas impermeable) package drug component, but it does not affect the drug contained therein.
A very common question in the industrial healthcare industry is what method of sterilization should I use for my product? I believe this is easily addressed by simply answering two key questions. The first question is what sterilization method(s) will work for my product? If the answer is that only EO will work, or only radiation will work, then your path is clear. In some cases it may turn out that you can use either EO or radiation. The second question then becomes which method will sterilize my product in the most cost-effective manner? Do keep in mind that when answering the second question, one must look at the “true” cost of sterilization, which includes many factors, and must take into account the entire supply chain for the sterilization process.
Editor's note: During the posting of this letter an error was made in which the editor posted "1350 degrees F" and "1400 degrees F" rather than the correct "135 degrees F" and "140 degrees F." The posting has been corrected and the editor apologizes for this error.